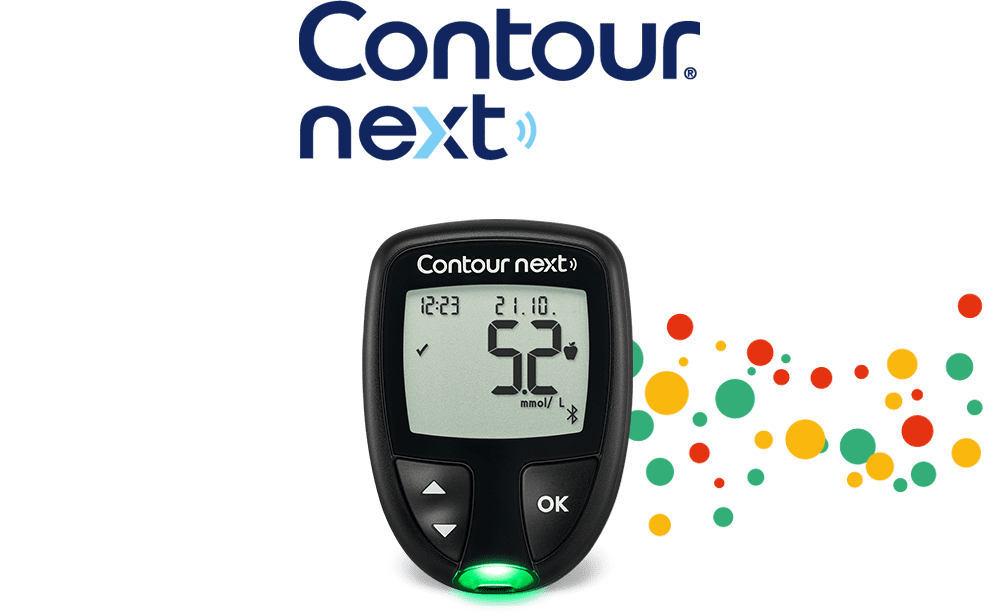
Highly accurate blood glucose data with the CONTOUR®NEXT blood glucose meter*†1,2

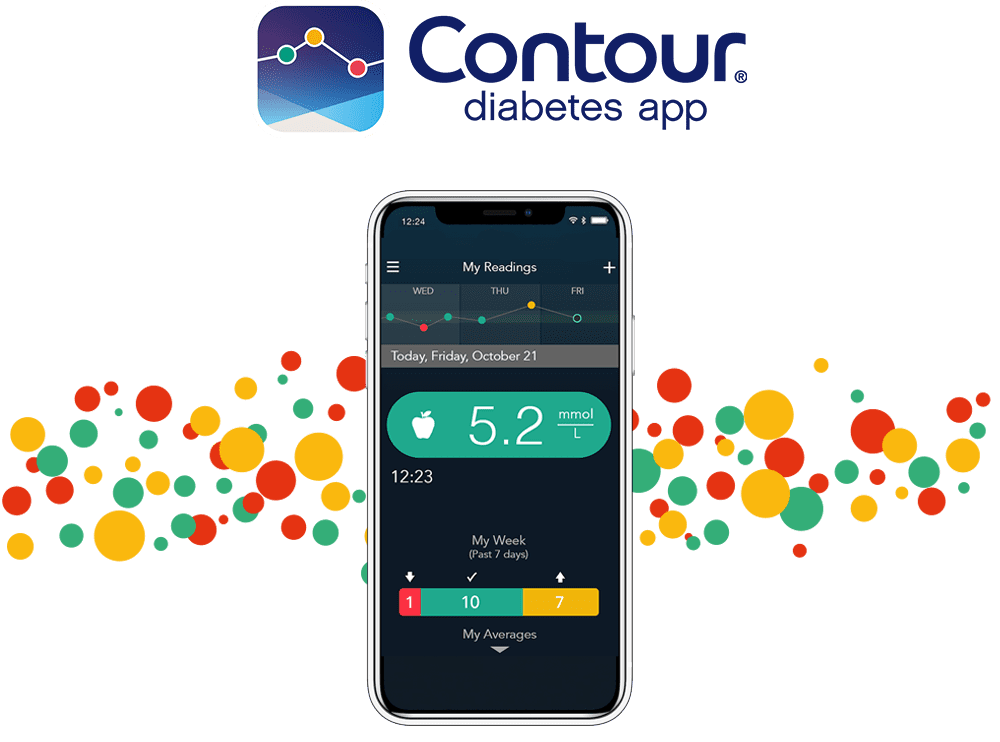
Convenient tools and a better understanding of blood glucose patterns with the CONTOUR®DIABETES app3


Share your blood glucose data seamlessly with healthcare professionals via GlucoContro.online**